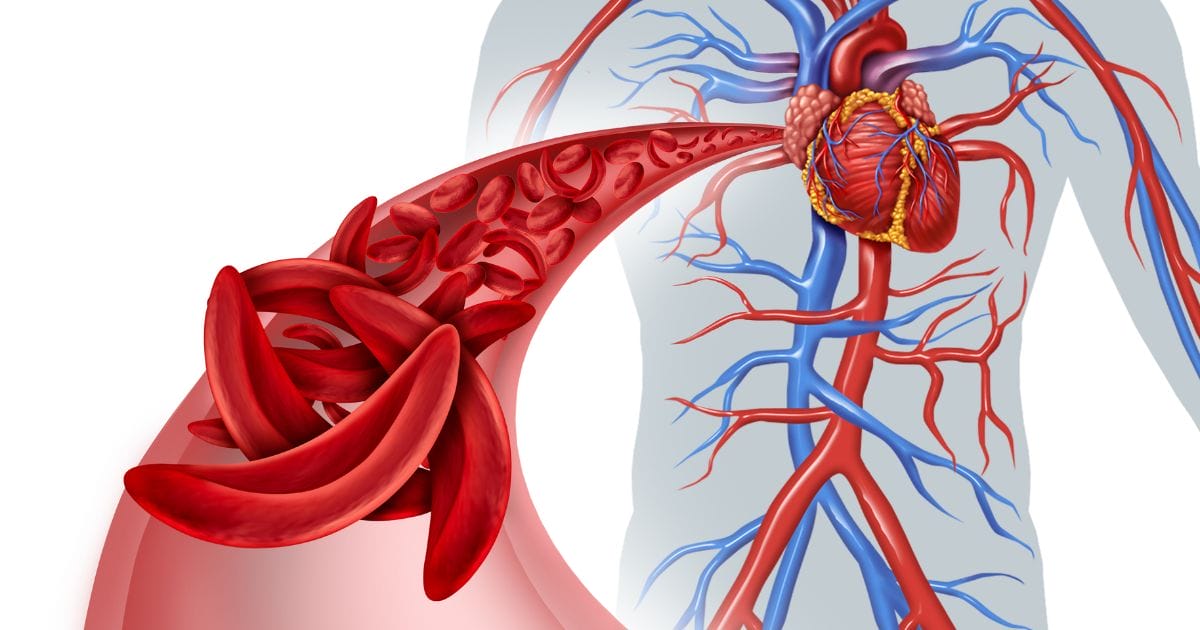
The Biden-Harris Administration announced recently that sickle cell disease (SCD) will be the first focus of the Cell and Gene Therapy (CGT) Access Model, which was initially announced in February 2023. The model is designed to improve health outcomes, increase access to cell and gene therapies, and lower health care costs for some of the nation’s most vulnerable populations.
Sickle cell disease is an extremely painful condition, which disproportionately impacts Black Americans and has had limited treatment options. In the United States, more than 100,000 people live with SCD. Individuals with the disease have a shorter life expectancy, by more than 20 years, compared to someone living without SCD. Additionally, many long-term health complications from SCD — including stroke, acute chest syndrome, and chronic end-organ damage — can lead to higher rates of emergency department visits and hospitalizations. Patients with SCD experience challenges with access to quality and affordability of care. This model has the potential to help improve health outcomes for patients and families with SCD while also ensuring taxpayer dollars are being used more effectively.
“HHS is using every tool available to us to increase access to high-quality, affordable health care and lower health care costs,” said HHS Secretary Xavier Becerra. “Many of the more than 100,000 Americans with sickle cell disease face difficulty accessing effective health care and groundbreaking treatments. While medical advancements bring us closer to cures, too many individuals with sickle cell disease and their loved ones still face challenges obtaining the care they need. With increased investment, we can improve the quality of life for people affected by this disease and find new, potentially transformative treatments.”
The CGT Access Model is part of the Administration’s broader effort to further drive down prescription drug costs and was developed in response to an executive order that President Biden issued in October 2022 directing the Department of Health and Human Services to consider developing models that increase access to novel therapies and lower the high cost of drugs. The model, led by the Centers for Medicare & Medicaid Services’ (CMS’) Innovation Center, will test outcomes-based agreements (OBAs) for groundbreaking CGTs. Successful OBAs will increase affordable access to potentially lifesaving and life-changing treatment. This model will begin in 2025 and may be expanded to other types of CGTs in the future.
“Gene therapies for sickle cell disease have the potential to treat this devastating condition and transform people’s lives, offering them a chance to live healthier and potentially avoid associated health issues,” said CMS Administrator Chiquita Brooks-LaSure. “Increasing access to these promising therapies will not only help keep people healthy, but it can also lead to savings for states and taxpayers as the long-term costs of treating sickle cell disease may be avoided.”
Approximately 50% to 60% of people living with SCD are enrolled in Medicaid. Hospitalizations and other health episodes related to SCD cost the health system almost $3 billion per year. Gene therapies for the treatment of SCD, as well as other complex conditions, hold significant potential to improve patient outcomes and therefore reduce long-term health spending, but they can also pose challenges to state budgets due to the high cost of the therapy.
Over the next year, CMS will partner with participating states and manufacturers to build a framework that expands access to gene therapies for the treatment of SCD. Under the model, CMS will negotiate an OBA with participating manufacturers, which will tie pricing for SCD treatments to whether the therapy improves health outcomes for people with Medicaid. Negotiations will also include additional pricing rebates and a standardized access policy. Participating states will then decide whether to enter into an agreement with manufacturers based on the negotiated terms and offer the agreed-upon standard access policy in exchange for rebates as negotiated by CMS. As part of the CGT Access Model, CMS will negotiate financial and clinical outcome measures with drug manufacturers and then reconcile data, monitor results, and evaluate outcomes. The CGT Access Model will begin in January 2025, and states may choose to begin participation at a time of their choosing between January 2025 and January 2026.
“The goal of the Cell and Gene Therapy Access Model is to increase access to innovative cell and gene therapies for people with Medicaid by making it easier for states to pay for these therapies,” said Liz Fowler, CMS Deputy Administrator and Director of the CMS Innovation Center. “By negotiating with manufacturers on behalf of states, CMS can ease the administrative burden on state Medicaid programs so they can focus on improving access and health outcomes for people with sickle cell disease.”
CMS anticipates addressing additional care delivery gaps and other hurdles for people receiving cell and gene therapy during the OBA negotiation process, including requiring manufacturers to include a defined scope of fertility preservation services when individuals receive gene therapy for treatment of SCD. CMS will also offer optional funding to states that engage in activities that increase equitable access to cell and gene therapies and promote multi-disciplinary, comprehensive care for people with Medicaid with SCD receiving gene therapy. These activities may include expanding or increasing reimbursement rates for optional Medicaid benefits and services, such as behavioral health or care management services.