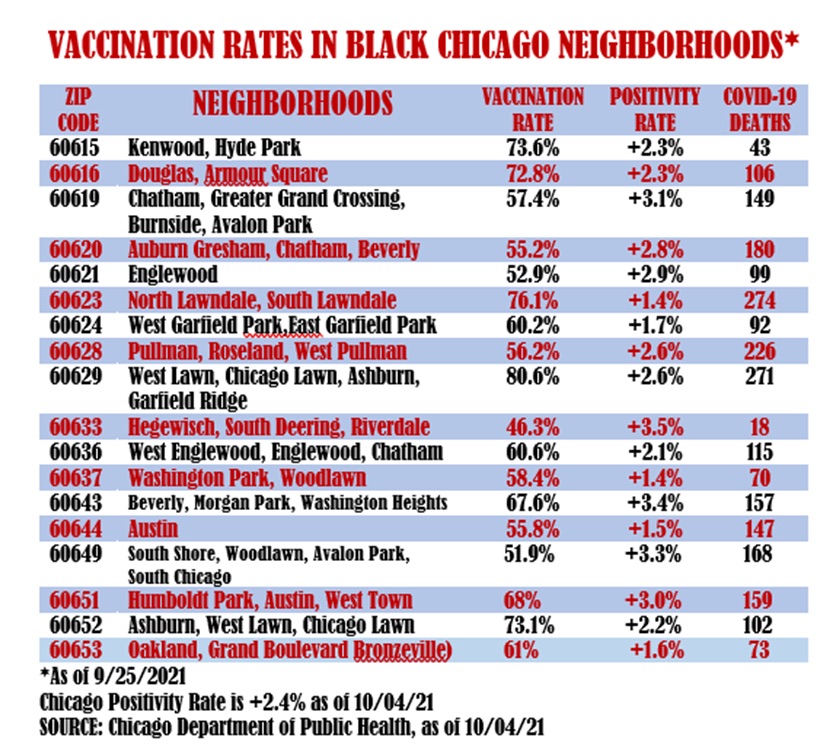
Englewood, the South Side neighborhood in Chicago that has long had the lowest vaccination rate in the entire city, is no longer at the bottom of the list, according to a Crusader analysis of data from the Chicago Department of Public Health.
The latest figures show that 52.2 percent of Englewood’s 29,042 population in zip code 60621 have received at least one dose of vaccine for COVID-19 as of September 25.
That percentage is higher than two zip codes on the South Side. Those zip codes are 60649, which is largely South Shore, and parts of Wood- lawn and Avalon Park. The other zip code is 60633, which includes Hegewisch, South Deering and Riverdale. In 60649, at least 51.9 percent of 46,024 residents there have received at least one dose of the vaccine. Zip code 60633 now has the lowest vaccination rate in the city at 46.3 percent.
Englewood has had the lowest vaccination rates since the city began re- leasing data on vaccination rates last December. The city and community groups have held numerous vaccination drives and projects to raise awareness of COVID-19 protection.
Out of 18 zip codes that the Crusader has maintained a database for during the pandemic, 60629, which includes Chicago Lawn, West Lawn and Ashburn, has the highest vaccination rate at 80.6 percent. Zip code 60623 (North Lawndale and South Lawndale) and 60615 (Kenwood and Hyde Park) are the second and third highest with 76.1 percent and 72.8 percent, respectively.
As of October 4, 63.2 percent of Chicago’s 2.7 million residents had received at least one shot of the vaccine. The city’s positivity rate for new cases early Monday, October 4, was at 2.4 percent while the state average stood at 2.7 percent. Out 18 Black zip codes monitored by the Crusader, nine had positivity rates lower than the city’s rates. Five zip codes had rates of 3 percent or higher.
With the lowest vaccination rate in the city, zip code 60633 had the highest positivity rate among 18 Black zip codes with 3.5 percent. The positivity rate in Englewood’s 60621 zip code stood at 2.9 percent. In zip code 60649, the positivity rate was at 3.3 percent.
With the holidays around the corner, health experts are already advising against family gatherings despite declining hospitalization rates across the country. Some fear gatherings might cause a surge in cases like the holiday season last year, where thousands of Americans died in January and February.
This week, Johnson & Johnson reportedly had plans to ask FDA officials to authorize a booster shot of its one-shot COVID-19 vaccine.
Many South Side residents in Chicago who received the Johnson & Johnson vaccine at Chicago State University are among the more than 15 million Americans who received the one-shot vaccine, according to the Centers for Disease Control and Prevention.
The FDA last week reportedly scheduled an October 15 meeting of its expert advisory committee to discuss whether to grant emergency use authorization for a booster shot of Johnson & Johnson’s vaccine.
But the biggest news in the battle against the virus is drugmaker Merck, who on October 1 made headlines after announcing that its experimental pill for people sick with COVID-19 reduced hospitalizations and deaths by half in trial efforts.
The results were so strong that an independent group of medical experts monitoring the results recommended ending the trial early.
Merck and Ridgeback Biotherapeutics said early results showed patients who received the drug molnupiravir—within five days of COVID-19 symptoms—had about half the rate of hospitalization and death as those who received a dum- my pill.
The study tracked 775 adults with mild-to-moderate COVID-19 who were considered high risk for severe disease because of health problems such as obesity, diabetes or heart disease. The results have not been reviewed by outside experts, the usual procedure for vetting new medical research.
Among patients taking molnupiravir, 7.3 percent were either hospitalized or died at the end of 30 days, compared with 14.1 percent of those getting the dummy pill. After that time period, there were no deaths among those who received the drug, compared with eight in the placebo group, according to Merck.
Merck only tested the pill on people who were not vaccinated. But FDA regulators may consider authorizing it for broader use in vaccinated patients who get breakthrough COVID-19 symptoms.
Patients take four pills of molnupiravir twice a day for five days. Side effects were reported by both groups in the Merck trial, but they were slightly more common among those who received a dummy pill.
If cleared by the Food and Drug Administration (FDA), the pill would be the first pill shown to treat COVID-19, and health experts hope it could help end the year-and-a-half-old pandemic.
“This would allow us to treat many more people much more quickly and, we trust, much less expensively,” Dr. William Schaffner, an infectious disease expert at Vanderbilt University who was not involved in the research, told the Associated Press.
National Chief Medical Advisor on Infectious Diseases Dr. Anthony Fauci called the results from Merck “very good news.”
Merck plans to ask the FDA for authorization soon. An approval could make the pill available soon afterward. Merck said it can produce pills for 10 million patients by the end of the year.
Despite the news of the pill, White House Coronavirus Coordinator Jeff Zients told the Associated Press that vaccinations will remain the government’s main strategy for controlling the pandemic.
“We want to prevent infections, not just wait to treat them when they happen,” he said.